sufficient supply of trace minerals and vitamins.
These salubrious circumstances have made nutri-
tional diseases uncommon and, consequently, make
laboratory evaluation of nutritional status an infre-
quent undertaking. A notable exception to this
generalization is the laboratory evaluation of the
nutritional status of the micronutrients involved in
red cell production: anemia due to impaired absorp-
tion of folate and cobalamin are often seen in
patients with intestinal malabsorption; anemia caused
by deficient absorption of cobalamin as a result of
intrinsic factor deficiency can be seen in the elderly;
and anemia due to iron deficiency is not rare in
infants and young children, in menstruating and
pregnant women, and in individuals with gastrointes-
tinal bleeding.
TRACE MINERALS AND VITAMINS
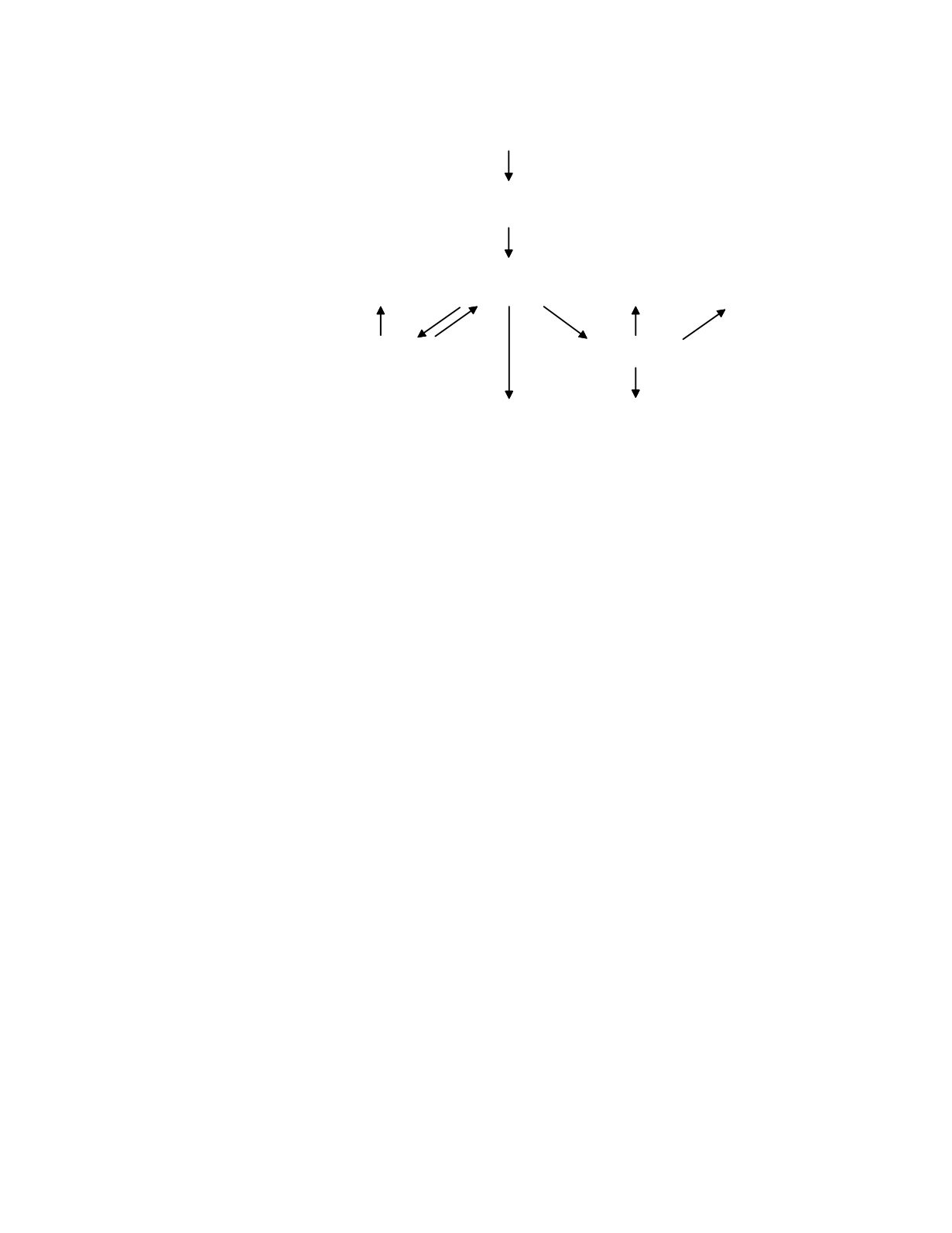
A simple model of the disposition of trace
minerals and vitamins is shown in Figure 8.1. The
tissues that utilize a trace substance receive their
supply of the substance from the circulation, where
it is available in its transport form, and from local
intracellular supplies of the substance. The transport
form arises from intestinal absorption of the
substance present in the diet and, if the trace
substance is stored, from the release of substance
from the storage form. The water-soluble vitamins
other than cobalamin are transported in the plasma in
their free form or loosely bound to albumin.
Vitamins in the free form are freely filtered at the
glomerulus and lost in the urine. For some
vitamins, notably pantothenic acid and biotin, this
can be the primary route of loss of the vitamin.
Most of the trace metals are transported in the
plasma bound to albumin. Iron, however, is bound
to an iron-specific binding protein, apotransferrin,
which releases the iron only after the iron-
apotransferrin complex (called transferrin) is bound
to cell surface transferrin receptors and taken up into
the acidic environment of early endosomes. This
facilitates the differential delivery of iron to tissues
expressing the transferrin receptor, such as red cell
precursors, placenta, and the liver, the storage organ
for iron. Cholecalciferol, cobalamin, and retinol are
also transported bound to specific binding proteins:
vitamin-D binding globulin, transcobalamin II, and
retinol-binding globulin, respectively. The
cobalamin-transcobalamin II complex (called holo-
transcobalamin II) enters cells by holo-
transcobalamin-specific
receptor-mediated
endocytosis. Thus, in a way similar to that of
protein binding of iron, the protein binding of
cobalamin directs the delivery of cobalamin to cells
requiring the micronutrient. The protein binding of
cholecalciferol and retinol allows both of these
vitamins, which are otherwise sparingly soluble in
water, to enter the circulation for delivery to tissues.
Because the dynamic equilibrium between the free
and protein-bound forms of these vitamins is so
much in favor of the bound form, very little of the
free form of either vitamin is available for diffusion
into most tissues. Tissues that possess high
Nutritional Status
8-2
storage tissues
active tissues
cell loss,
catabolism
gastrointestinal
absorption
urinary loss
diet
INTAKE
LOSS
PLASMA AND
EXTRACELLULAR FLUIDS
transport
form
TISSUES
storage
form
supply
form
product
form
Figure 8.1
A model of the disposition of trace minerals and vitamins.


