subject to swings in concentrations as a result of
irregularities in the dietary intake or synthesis of the
substance or of a precursor. The protein binding of
the thyroid hormones and of cholecalciferol may
serve this function. Consider, for example, that a
temporary deficiency in iodine intake with conse-
quent impairment of the synthesis of the thyroid
hormones could be partially ameliorated by the
transfer of TBG-bound hormone to the bioactive
fraction. Similarly the effect of seasonal variations
in cholecalciferol production upon the synthesis of
1,25-(OH)
2
D
3
the could be blunted by the reservoir
of 25-(OH)D
3
maintained bound to DBG.
The bioactive fraction
When referring to the effects of plasma protein
binding, the bioactive fraction of a blood constituent
is that fraction which participates in physiologic
processes when passing through a vascular bed. Its
magnitude depends largely upon the distribution of
the constituent among its unbound and protein-bound
forms. This distribution is determined by the affin-
ity of the binding proteins for the substance and the
plasma concentration of the binding sites, i.e., the
capacity of the binding proteins. In the case of a
single binding protein, the distribution satisfies the
simplified equilibrium mass action equation,
k
a
[
free binding sites
] =
[
bound substance
]
[
unbound substance
]
where
k
a
is the association constant. (The associa-
tion constant is equal to the rate constant of associa-
tion of the substance and the binding protein divided
by the rate constant of dissociation of the complex).
Inspection of the equation reveals that the ratio of
bound to unbound substance is large when the
association constant is large (high affinity binding)
or if the concentration of binding protein, and hence
free binding sites, is large (high capacity binding).
Conversely, the ratio is small if there is low affinity
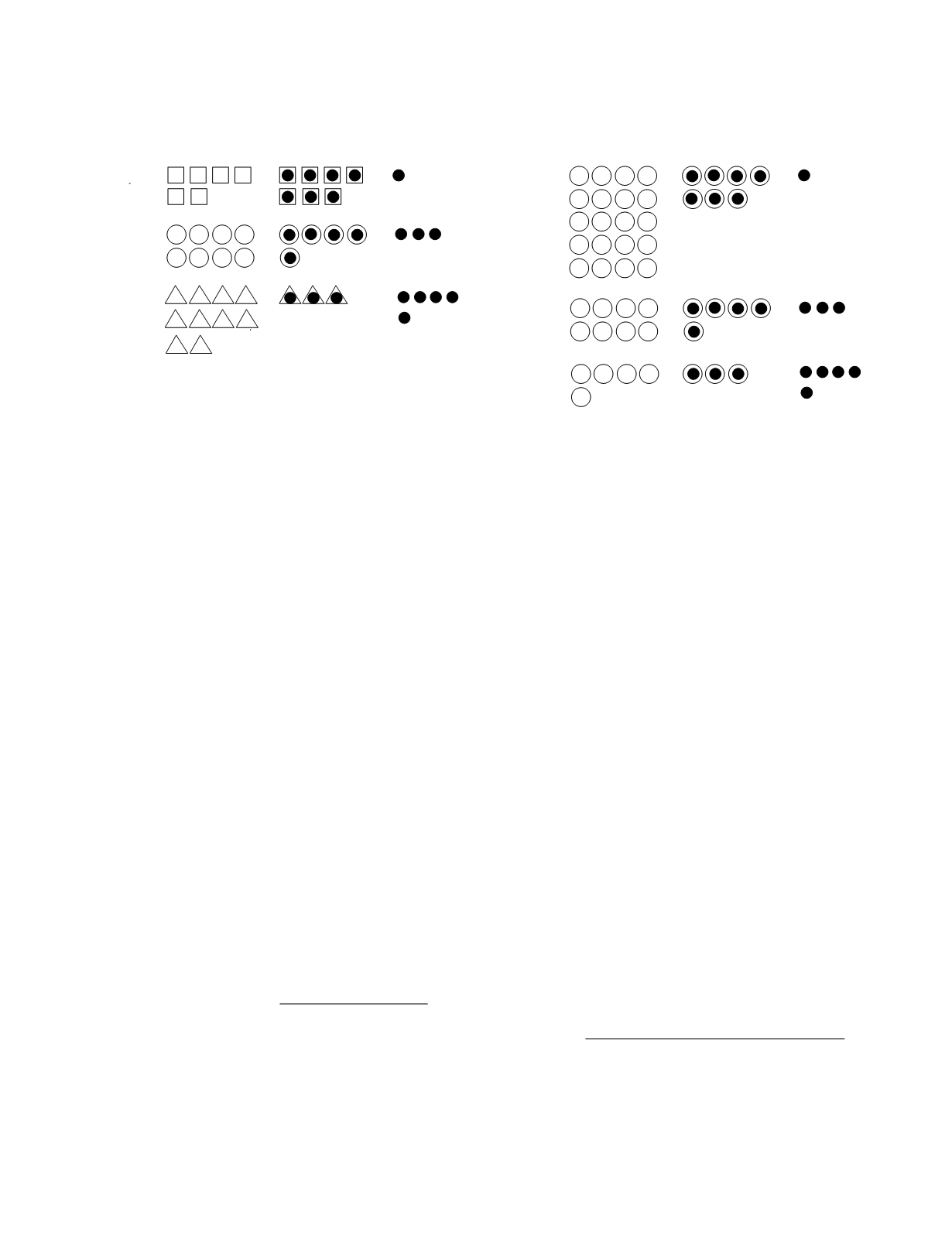
or low capacity binding. Figure 7.12 shows the
partition of a constant amount of a ligand between
protein-bound and unbound forms for three binding
proteins of equal capacity but unequal binding affin-
ity. In this example, the association constants are
1.17, 0.21, and 0.06 for the high-, moderate-, and
low-affinity binding, respectively. The effect of
varying the capacity of a binding protein (association
constant, 0.21) is depicted in Figure 7.13.
The relative affinities and capacities of the
plasma proteins binding the thyroid and steroid
hormones are listed in Table 7.2. Notice that the
hormones may bind to a number of proteins and that
each binding protein may associate with more than
one hormone. Calculating the distribution of these
hormones among their protein-bound forms is not at
all easy. Using computer-based techniques, the
distribution can be calculated by solving a system of
general equilibrium mass action equations (Feldman
et al.
1972); for
i = 1, . . . , n
2
[
bound substance
i
] =
j
=
1
n
j
k
ij
[
binding site
j
][
unbound substance
i
]
1
+
g
=
1
n
2
k
gj
[
unbound substance
g
]
where
n
1
is the number of binding sites,
n
2
is the
number of ligands, and
k
ij
is the association constant
of the
i
th ligand. Equilibrium binding distributions
Organ Function
7-13
high
capacity
moderate
capacity
low
capacity
unbound
binding sites
bound
sites/ligand
unbound
ligand
Figure 7.13
The effects of binding protein capacity upon the
distribution of a substance between its bound and unbound
forms.
Figure 7.12
The effects of binding protein affinity upon the
distribution of a substance between its bound and unbound
forms.
high
affinity
moderate
affinity
low
affinity
unbound
binding sites
bound
sites/ligand
unbound
ligand


