Screening
The rationale for clinical screening programs is
the detection of treatable disorders prior to their
becoming clinically manifest. In consideration of
the criterion for being treatable, this translates, at a
minimum, into the detection of cancer before it has
become metastatic and often it translates into the
detection of cancer while it is still limited to the
organ of origin. In order to achieve this end, the
screening study must have acceptable diagnostic
performance during some portion of the early phase
of the growth of the cancer
—
that is, it must have a
screening window
—
and the screening program must
be designed so as to assure that the screening study
is performed at least once during that screening
window. The likelihood of a screening marker
achieving the first goal depends upon the natural
history of the cancer and the pattern of the marker’s
expression within the context of that natural history.
The likelihood of a screening program achieving the
second goal depends upon the schedule on which the
screening study is performed. These points will be
illustrated by a consideration of screening for
prostate cancer using PSA. PSA will also serve as
the illustrative marker in several subsequent sections
of this chapter. There is no small degree of contro-
versy surrounding the use of PSA as a screening
marker (Woolf and Rothemich 1999, Svetec and
Thompson 1998, Moss and Melia 1998). The reader
is strongly urged to refer to the cited articles as well
as to more recent articles in the literature to appreci-
ate the many viewpoints in this controversy.
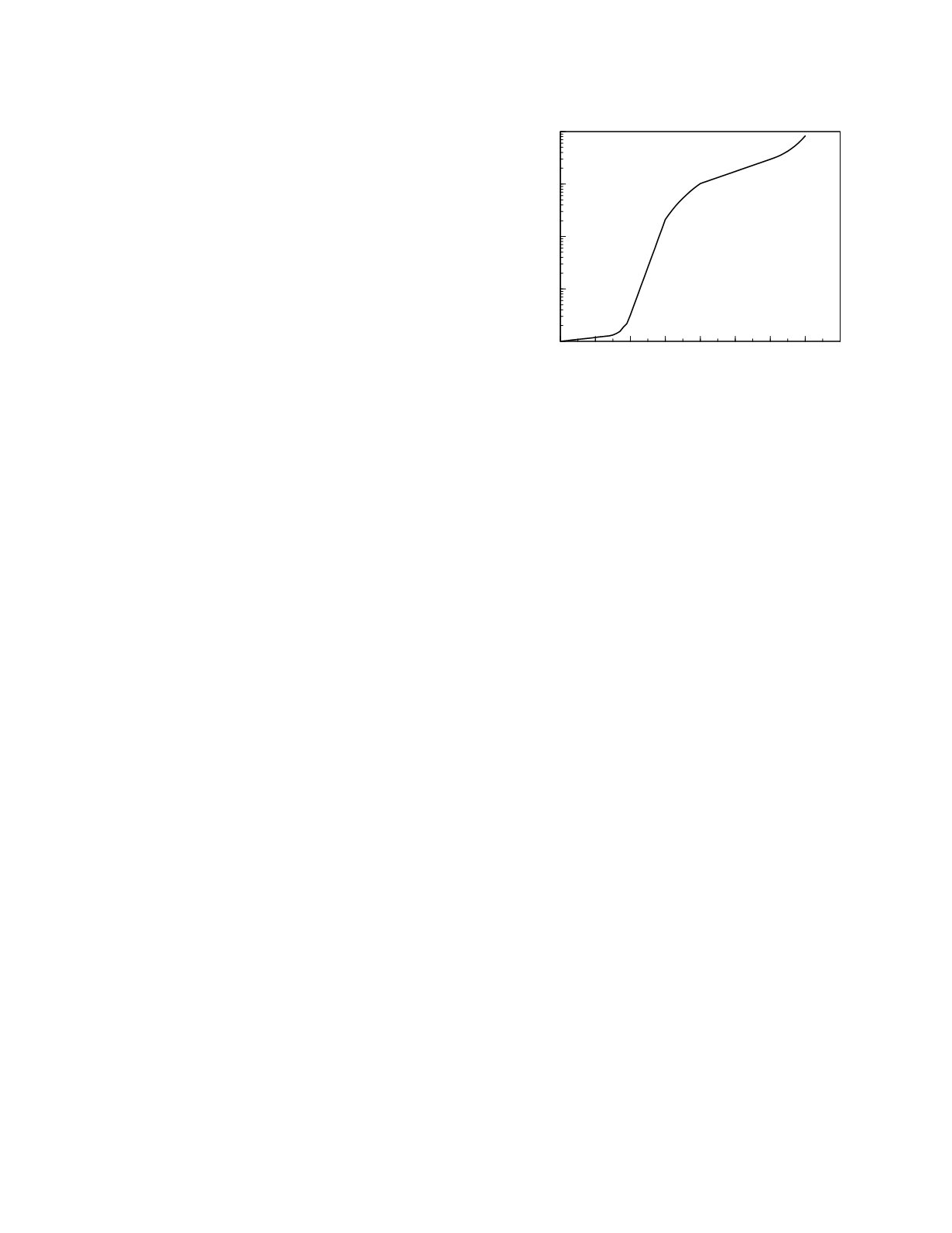
The natural history of prostate cancer, according
to the model proposed by Stenman
et al.
(1999), is
depicted in Figure 11.2. There is an initial phase of
intraepithelial neoplasia that can arise as early as the
third or fourth decade of life (Franks 1954) but
which may appear later in life. During this phase,
which is of uncertain duration (in the figure, a
duration of 15 years is shown), the cancer has a
fairly slow rate of grow. Once the tumor becomes
locally invasive it experiences a period of relatively
rapid growth. When the cancer reaches a size at
which vascularization is needed, its growth rate
lessens with doubling times estimated to be on the
order of two to three years (Schmid
et al.
1993).
This rate of growth persists until the cancer
metastasizes.
Before prostate cancer becomes metastatic, the
rate of entry of PSA into the body fluids appears to
be directly related to the size of the cancer. A direct
relationship between size and PSA entry rate is also
found for benign prostatic hyperplasia (BPH). The
magnitude of the entry rates differs considerably
between the two diseases, however. Stamey
et al.
(1987) estimate that, on average, normal prostate
contributes to plasma PSA concentration at a rate of
0.2 µg/L/g, BPH at a rate of 0.6 ug/L/g, and
prostate cancer at a rate of 2 µg/L/g. Statistical
modeling of the large data set in the report by
Collins
et al.
(1993) indicates that BPH increases
plasma PSA concentrations with a median value of
0.13 µg/L/g and that normal prostate makes only an
extremely small contribution (Noe, unpublished;
based on a model of direct proportionality between
tissue volume and plasma PSA concentration and a
lognormal distribution of PSA values). Modeling of
the data in Partin
et al.
(1990) yields median values
of 0.14 µg/L/g and 2.64 µg/L/g, respectively, for
BPH and prostate cancer (Noe, unpublished).
In men with prostate cancer but otherwise
normal prostates, prostate cancer would be detect-
able as soon as the plasma PSA concentration
reached the limit of detection of the assay used to
measure PSA concentration. A desirable value for
this limit is 0.2 µg/L (Stenman
et al.
1995). Based
on a median value of 2.64 µg/L PSA concentration
rise per 1 g of cancer tissue, half of the men would
have detectable PSA concentrations while their
cancers weighed 0.075 g or less. If the standard
deviation in the lognormal distribution of the
relationship between PSA concentration and tissue
mass in prostate cancer is similar to that in BPH,
ninety-five percent of the cancers would be detect-
able while their mass was less than 0.22 g. This is
well shy of the mass associated with capsular
Cancer
11-7
Figure 11.2
A model of the development of prostate
cancer.
0 10 20 30 40 50 60 70 80
Years of development
0.01
0.1
1
10
100
Tumor diameter (mm)


