clinicians. Additionally, quality goals can be
derived from a consideration of the biologic variabil-
ity of the analyte being measured. For instance, as
discussed in Chapter 1, the extent of the variability
in study results with repeated testing of an individual
is determined by the within-individual biologic
variability of the analyte and the within-laboratory
analytical variability,
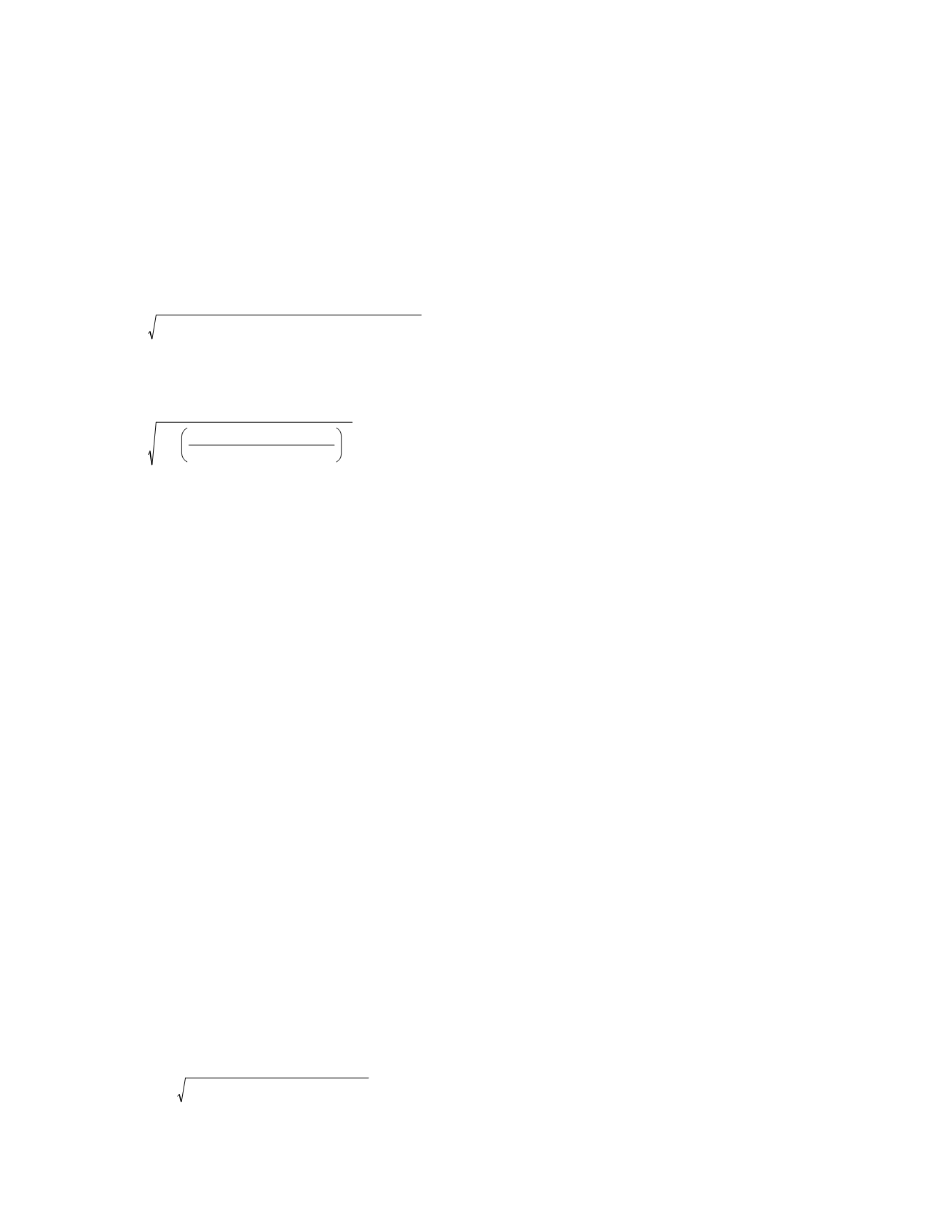
SD
within-individual
=
SD
within
−
individual
,
biologic
2
+
SD
within
−
laboratory
2
The fractional increase in the total within-individual
variability attributable to analytic variability is,
therefore,
1
+
SD
within
−
laboratory
SD
within
−
individual
,
biologic
2
−
1
To keep the contribution of the analytical component
at a reasonable level, say 10 percent, the ratio of the
within-laboratory variability to the within-individual
biologic variability must be less than 0.459. Round-
ing up to 0.5 (for which the fractional increase in
within-individual variability is 11.8 percent) yields
the quality goal for repeated testing (Cotlove
et al.
1970, Harris 1979, Fraser
et al.
1997),
SD
within-laboratory
< 0.5 SD
intra-individual,biologic
This rule can also be expressed as
CV
within-laboratory
< 0.5 CV
intra-individual,biologic
which is particularly useful if within-individual
biologic variability is proportional to analyte concen-
tration. Then both coefficients of variation will be
constant.
The reference interval for an analyte depends
upon the median analyte value and the total (intra-
and inter-individual) biologic variability of the
analyte. In also depends upon the bias in the
measurement of the analyte and the within-
laboratory analytical variability. When calculated
based on the assumption of a normal frequency
distribution,
reference interval =
median value + bias ± 1.96 SD
total
where
SD
total
=
SD
biologic
2
+
SD
within
−
laboratory
2
The presence of bias results in a displacement of the
measured reference interval from the true reference
interval which is,
median value ± 1.96 SD
biologic
As a result, at one side of the reference interval,
individuals who fall inside the measured reference
interval fall outside of the true reference interval.
At the other side of the reference interval, individu-
als who are outside of the measured reference inter-
val are inside the true reference interval. The
fraction of the population misclassified in this way
should be kept to an acceptable level. If a 5 percent
misclassification rate is used as the standard, in the
absence of analytical imprecision, the ratio of the
bias to the total biologic variability should be kept
less than 0.315. Rounding down to 0.25 (for which
the misclassification rate equals 3.7 percent) yields
the quality goal for reference intervals (Gowans
et
al.
1988 and 1989, Fraser
et al.
1997),
bias < 0.25 SD
biologic
which can also be expressed as
relative bias < 0.25 CV
biologic
Analytic imprecision widens the reference interval
and thereby also results in misclassification. Using
3.7 percent misclassification as the standard, in the
absence of bias, the ratio of the within-laboratory
variability to the total biologic variability should be
kept less than 0.56. This is another quality goal for
reference intervals (Fraser
et al.
1997),
SD
within-laboratory
< 0.56 SD
biologic
which can also be expressed as
CV
within-laboratory
< 0.56 CV
biologic
Of course, the misclassification rate should also be
within desirable limits when both bias and impreci-
sion are present. Figure 2.3 shows the paired values
for relative bias and imprecision that satisfy the 3.7
percent misclassification standard.
The application of these quality goals can be
illustrated by using them to define the desirable
analytical quality of a field method for plasma creati-
nine concentration. At a concentration of 100
µmol/L, the average SD
within-individual,biologic
is 4.3
µmol/L (CV
within-individual,biologic
, 4.3%) and the average
SD
biologic
is 11.3 µmol/L (CV
biologic
, 11.3%)
(Sebastián-Gámbaro
et al.
1997). The quality goal
for precision based on the rule for repeated testing is
an SD
within-laboratory
of less than 2.15 µmol/L
Laboratory Methods
2-7


