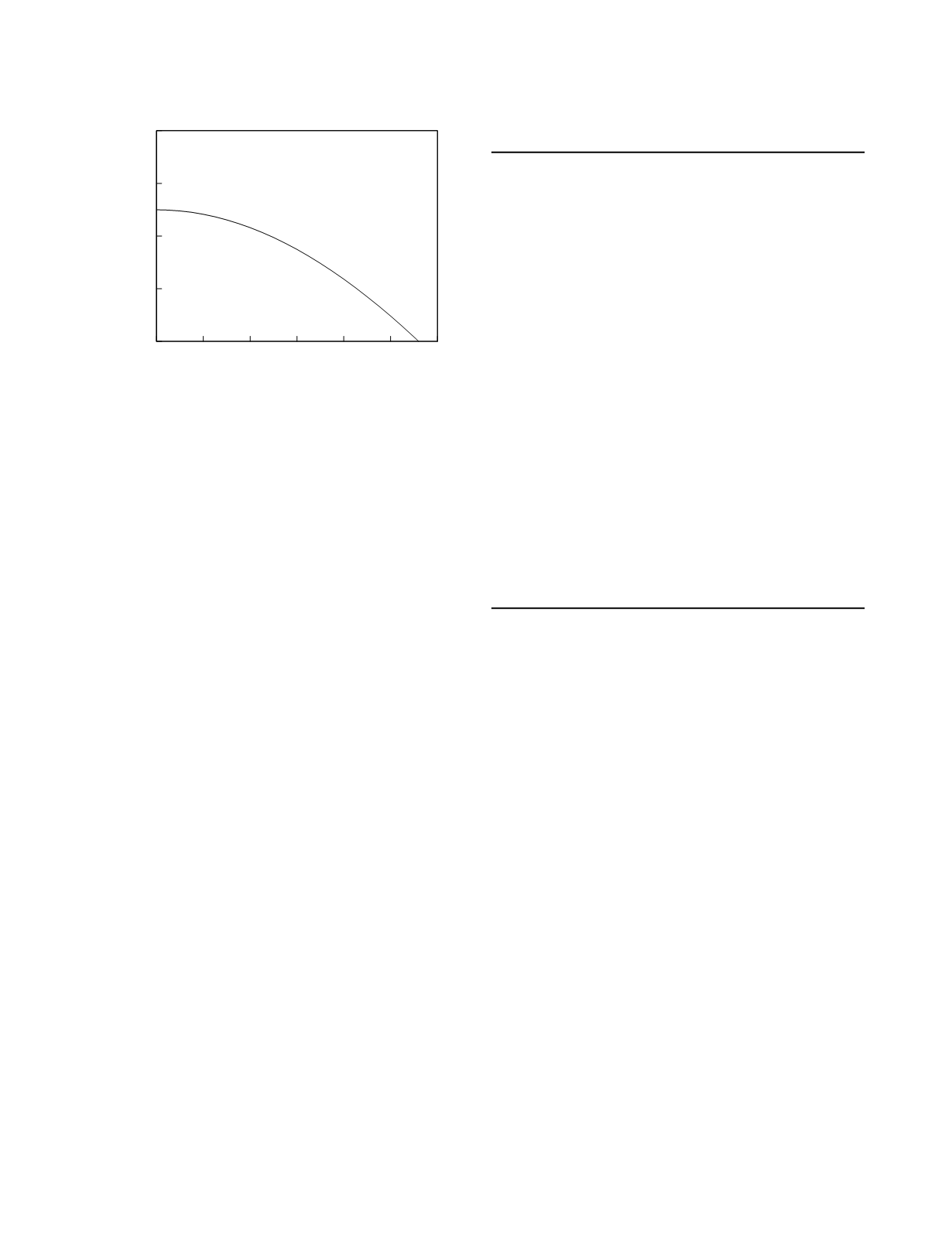
(CV
within-laboratory
of less than 2.15%). At this level of
imprecision, the ratio of SD
within-laboratory
to SD
biologic
is
0.19. So, using Figure 2.3, the quality goal for
trueness based on the rule for reference intervals is a
relative bias of less than 0.215 which corresponds to
an absolute bias of less than 2.43 µmol/L.
MAINTAINING QUALITY
Once a laboratory method has been implemented
in the clinical laboratory, its quality is maintained by
strict adherence to the approved procedure for
performing the method, by the maintenance of high
technical skill among the method operators, by the
regular maintenance of the instruments utilized in the
method, and by a rigorous quality assurance
program.
Written measurement procedure
The laboratory document that contains the
description of the steps in the performance of a
method is called the written measurement procedure
(Dybkaer 1997). In addition to describing the
method, this document includes introductory
material, a description of the quality assurance
program for the method, and a summary report of
the quality evaluation of the method (Table 2.3).
The introductory material identifies the method,
summarizes the clinical rationale for the measure-
ment of the analyte and the laboratory rationale for
the choice of method, specifies the type of specimen,
and stipulates safety precautions in the use of the
method. The introductory material also provides
lexicographic support for an unambiguous reading of
the method description and lists of cited and recom-
mended references. The procedure is periodically
reviewed and is abridged as necessary. The dates of
review and the dates and details of changes in the
method are recorded and filed with the introductory
material.
The method description is thorough and detailed.
It includes sections devoted to specimen collection
and handling, reagents and equipment, preparation
and performance of the method, calculation of
results, and quality assurance. Aspects of specimen
collection to be considered include any special
preparation of the patient for the taking of a speci-
men and the identification of the collection device
and the specimen container. The handling of the
specimen is detailed as regards anaerobic conditions,
temperature, the allowable time prior to processing,
the method of processing, and the conditions of
storage of the processed specimen. The list of
reagents stipulates the identity and source of each
reagent and gives instructions for its storage,
handling, and disposal. The preparation of stock
and working solutions is described and the
Laboratory Methods
2-8
0
0.1 0.2 0.3 0.4 0.5 0.6
SD
within-laboratory
/ SD
biologic
0
0.1
0.2
0.3
0.4
Bias / SD
biologic
Figure 2.3
Analytical quality goals for method imprecision
and method bias based on a consideration of patient classi-
fication using the reference interval for an analyte. Impreci-
sion and bias are expressed relative to total biologic
variability.
Table 2.3
Components of a Written Measurement Procedure
Introductory material
Title
Table of contents
Introduction
Scope
Warning and safety precautions
Definitions
Symbols and abbreviations
References
Dates
Method description
Sampling and specimen handling
Principle of measurement
Reagents
Apparatus
Preparation of measurement system
Use of measurement system
Modifications of the usual procedure for special cases
Calculation of results
Quality assurance program
Analytical performance description
Analytical quality evaluation findings
Method comparison findings


