The quality of category IV and V diagnostic
performance evaluations and category 2 and 3
prognostic performance evaluations is assessed
according to the design standards described in the
preceding section of this chapter. Some studies may
be found to have one or more serious design flaws
which bring into question the validity of the evalua-
tion findings. These studies should be considered
unacceptable and they should be excluded from
further analysis. Studies with less serious flaws may
be grouped and analyzed separately or, if they are
included in the quantitative meta-analysis, may have
their contribution to the analysis weighted by some
index of quality. No standard quality weighting
index exists, to date. All well-designed performance
evaluations should be included in the quantitative
meta-analysis.
Quantitative meta-analysis attempts to combine
the quantitative findings of performance evaluations
in a way that will give a more complete and
presumably more accurate representation of the
performance of a laboratory study. This is done by
combining the report results so as to generate aggre-
gate performance data. How this is accomplished
depends upon the presentation of the individual
evaluation results. When complete performance data
are available in the form of result frequency distribu-
tions, the frequency data can be combined so as to
produce aggregate result frequency distributions
from which aggregate ROC and likelihood ratio
Evaluating Classification Studies
4-10
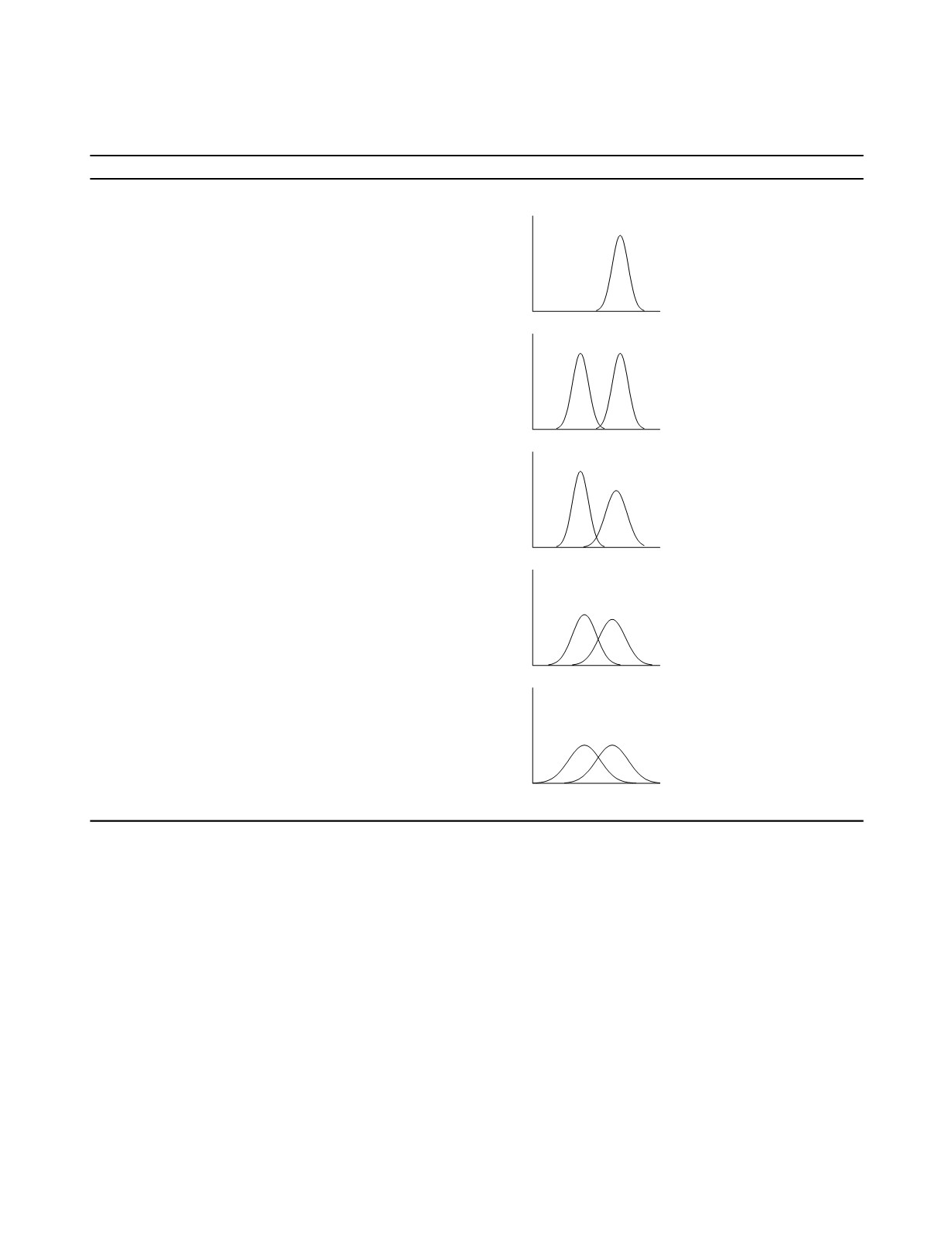
Table 4.3
Categories of Performance Evaluation Design
Category Characteristics
Example study result distributions
I
performance of procedures
cases: typical spectrum of disease
controls: none
II
coarse distinctions
cases: typical spectrum of disease
controls: healthy
III
more subtle distinctions
cases: expanded spectrum of disease
controls: healthy
IV
preliminary clinical application
cases: include appropriate comorbidity
controls: include appropriate comorbidity
V
definitive clinical application
cases: full spectrum
controls: full spectrum


